Radiosurgery for Male Patients with Breast Cancer Brain MetastasesKeywords: brain metastasis, brain tumor, gamma knife, breast cancer, radiosurgeryInteractive ManuscriptAsk Questions of this Manuscript: What is the background behind your study?Male breast carcinoma (MBC) is extremely uncommon, with incidence of only 1.2 cases per 100,000 men. Management of such rare brain tumors is not well characterized. What is the purpose of your study?The purpose of this study was to understand the role of stereotactic radiosurgery (SRS) in the management of brain metastases from MBC. Describe what you did.We reviewed records from 3 patients who underwent SRS for the management of their brain metastases, selected from a group of over 4,000 patients with all types of brain metastases managed over a 23 year interval. Describe your patient group.This question was not answered by the author Describe your main findings.In this case report three patients were treated for a total of 4 metastases. Treatment resulted in regression of 2 metastases, no change in 1 metastasis, and progression in 1 metastasis. Median survival time following SRS was 39 months, with a range of 4-47 months. Describe the main limitation of this study.The main limitation of this study was the small sample size. Describe your main conclusion.Stereotactic radiosurgery was an effective treatment in patients with brain metastases from male breast cancer. Describe the importance of your findings and how they can be used by others.Male patients with breast cancer tend not to do worse than a comparable cohort of female patients treated with SRS. Brain metastases from breast cancer may be managed with SRS similarly in males and females. What is the background of your topic?
Male breast carcinoma (MBC) is extremely uncommon, accounting for less than 1% of all cancer in men. Approximately 2,000 new cases are diagnosed in the United States each year, 23 with an incidence of just 1.2 per 100,000 men. 12 The etiology of MBC has been linked to BRCA 1 and BRCA 2 mutations, as well as Klinefelter’s syndrome and several acquired and environmental factors. 1
Brain metastases develop in 20-40% of patients with cancer, and are a significant cause of morbidity and mortality. 14 Due to the rarity of MBC and limited data, clinical management is primarily based on the management of female breast carcinoma (FBC). Treatment modalities for brain metastases from primary breast carcinoma in both males and females include surgical resection, whole brain radiation therapy (WBRT), and more recently, stereotactic radiosurgery (SRS).
What is the importance to the reader/community?
There have been no prior reports of the management of CNS metastases from MBC for multiple patients. What is your hypothesis? (What question(s) did you ask?)Therefore, we sought to determine whether SRS was an effective treatment for brain metastases from MBC. What was done in your study?
We reviewed three cases of patients with brain metastases from MBC
who were treated with SRS. What are the main conclusions derived from prior reports?
Prior reports have suggested that outcomes for males are typically similar to female patients, however there has only been limited data in case report format on male patients with brain metastases. What are the main limitations of your research method?The main limitation of this study was the small sample size. If your work has Institution Review Board or any other supervisory authority approval, state that now:
This retrospective study was approved by the University of Pittsburgh Institutional Review Board. State the source of funding for this study.This work was not funded by an external source. Describe patient age (mean, range)At the time of SRS, patient 1 was 51 years old, patient 2 was 67 years old, and patient 3 was 70 years old. Genetic testing was not performed on these patients. Describe patient sex (number male and number female)All patients were male. Describe other important patient features (symptoms (list); clinical presentation features, prior treatment, employment, etc)Patient 1 was diagnosed with primary MBC 4 years before SRS. At the time, the patient underwent a mastectomy and axillary node dissection. Nearly four years before SRS, the patient was found to have a solitary brain metastasis in the left cerebellar region, which was treated with WBRT. A scan performed one month before SRS revealed progression of the cerebellar tumor along with a small new metastasis in the medial right temporal lobe. At the time of SRS, the patient was neurologically asymptomatic.
Patient 2 was diagnosed with primary MBC 7 years before SRS. The primary site was managed with a right modified radical mastectomy, chemotherapy (Cytoxan and Adriamycin, followed by Tamoxifen then Arimidex), and radiation therapy. He presented to the SRS clinic with right-sided lip and chin numbness and partial diabetes insipidus. Magnetic resonance imaging (MRI) revealed enlargement of the pituitary gland, as well as multiple areas of T2 prolongation throughout the cervical spine and clivus, consistent with metastatic disease from primary breast cancer. There was no noticeable involvement in the cavernous sinus to explain the right V3 numbness. The patient was placed on DDAVP to control the diabetes insipidus, and underwent SRS to the pituitary.
Patient 3 was
diagnosed with primary MBC 8 years before SRS. The primary site was treated with a left modified radical mastectomy, followed by a six-month adjuvant chemotherapy regimen (Cytoxan, Methotrexate, 5-FU, Tamoxifen and Prednisone). The patient was disease-free and asymptomatic until 5 years before SRS, when he began noticing flashing lights in the left visual field. Computed Tomography (CT) imaging revealed a solitary right occipital tumor. This was surgically resected, followed by WBRT (50 Gy in 25 fractions). Post-operatively, his visual field symptoms persisted and the patient developed ataxia. At the time of SRS, MR imaging revealed a small solitary recurrent occipital metastasis.
All patients had physical exams consistent with Karnofsky Performance Status (KPS) >70 at the time of SRS. History of genetic testing for mutations associated with increased risk of breast cancer was unavailable.
Describe disease features (example = tumor subtypes)Tumor histologic types varied. Patient 1 had adenocarcinoma of unknown stage, histologic grade, or hormone status. Patient 2 was staged T2N1M0, with a histology of infiltrating, moderately differentiated ductal carcinoma, ER+/PR+. Patient 3 was staged
T2N1M0, with a histology of poorly differentiated, infiltrating lobular carcinoma, ER+/PR+, with tumor present in four axillary lymph nodes. Describe the clinical intervention (ie. medications, devices, techniques)
Gamma knife stereotactic radiosurgery was performed in all three patients (Elekta, Norcross GA). Patient 1 received 15 Gy to the margin of his cerebellar tumor, which measured 2.9 cc (maximum dose 30 Gy) and 15 Gy to the margin of his temporal lobe tumor, which measured 1.9cc (maximum dose 21.43 Gy). Patient 2 received 16 Gy to the margin of a 1.3cc tumor in the clivus (maximum dose, 32 Gy) (Figure 1). The maximum dose to the optic nerve was 8 Gy. Patient 3 received 20 Gy to the margin of his 0.3cc occipital tumor (maximum dose 40 Gy). No patient underwent additional whole brain radiotherapy or surgical resection after receiving SRS.
Describe the tests used to perform your research (Imaging, Patient Outcomes, Other specific tests.)Changes in tumor size were determined by contrast-enhanced MR imaging for all patients, and symptomatic changes were determined by clinical correspondence. What percent of study subjects completed each of the tests?All subjects had both clinical and imaging followup after SRS. Describe who conducted the tests. (Study investigators or other parties?)The follow-up assessments were evaluated by clinicians not involved directly in patient care. Were the tests validated for use in this kind of study?The outcomes measures were validated for this study. Describe your statistical methods or tests usedNo statistics were calculated, as only three patients were in the study. Describe your study power calculation (if any)This study did not include a power calculation. Describe your chosen level of statistical significanceDue to the small sample size, no statistical comparisons were performed. Provide the results for the most important outcome of your research [i.e. Patient survival]We followed patients serially with imaging and clinical assessments. Overall patient survival is shown in Data Share 2. The survival of this small series varied widely. Patient 1 died 39 months after SRS due to CNS progression. Patient 2 died 4 months after SRS, possibly due to CNS progression. Patient 3 died 47 months later due to unknown causes. Discuss any additional outcomes of your study [i.e. Imaging findings, Patient functional outcomes, Complications]Clinical Response
The clinical response to radiosurgery is shown in Data Shares 3-5. In this report, we analyzed the response to symptoms present at the time of SRS. Patient 1, who initially presented without symptoms, began to experience short-term memory loss over the course of 2-3 years following SRS, likely due to progression of the tumor in the right temporal lobe. Patient 2, who presented with facial numbness in the V3 region and partial diabetes insipidus, experienced increased numbness one month after SRS. The numbness resolved within one week after administration of corticosteroids to control edema. The patient also developed full central diabetes insipidus, which was controlled with continued oral DDAVP. Patient 3, who initially presented with ataxia and a left visual field cut, initially experienced symptom resolution 6 months following SRS and became completely asymptomatic 10 months following SRS.
Imaging Response
The imaging response to radiosurgery is shown in Data Shares 6-8. Patient 1, who received SRS for both a right temporal tumor and a left cerebellar tumor, showed regression of both tumors for the first 22 months after SRS. Imaging performed 30 months after SRS showed almost no residual tumor in the cerebellum, but significant enlargement of the right temporal tumor, which had a diameter increase from 1.3cc at 22 months to 3.0cc at 30 months. The patient received no treatment for the right temporal progression. Patient 2, who received treatment for a pituitary metastasis, showed a stable tumor one month following SRS (Figure 2). However, he had a new tumor in the choroid plexus. The patient was subsequently lost to follow-up and received no treatment for the new metastasis. Patient 3, who received treatment for a small solitary right occipital metastasis, showed tumor regression 3 months following SRS, followed by complete disappearance 10 months after SRS.
Discuss Statistical Outcomes [i.e. Multivariate analyses]Because of the small sample size, multivariate testing was not performed. Provide the background and reason for your work and briefly summarize important prior research.
CNS involvement due to male
breast cancer drastically affects both the treatment options and prognosis for
patients. 4,6,8,15 There are several notable differences between MBC
and FBC. For example, MBCs are mostly ductal in origin, 1,20 and 90.6% of MBCs are ER+, compared to 76.0% of FBCs.
Similarly, 81.2% of MBCs are PR+ compared to 66.7% of FBCs. 1,10
HER-1 positivity has been reported as ranging from as low as 1.1% 3
to as high as 37% in men, 10 compared to approximately 26-27% in
women. 1,3 Arslan et al. 2 reported in their non-metastatic male breast cancer series
(N=118), the proportions of positivity of estrogen receptor (ER),
progesterone receptor (PGR), and HER2 status were 82.9, 75.8, and 23.4%,
respectively. In our previous FBC SRS series (N=350), the proportion of
positivity of ER and HER2 status were 50 and 59%, respectively. 14 Additionally, MBCs present an average of 10 years after
FBCs. 1,5,7,13
All studies that have
considered the management of CNS metastases from breast cancer consisted of
entirely female or combined male and female populations. It was shown that the
risk of developing brain metastasis from breast cancer in a combined male and
female population is increased in patients with the following characteristics:
a younger age; a higher histological grade; estrogen receptor positive,
progesterone receptor positive, and HER2 negative > triple negative status
> HER2 positive; a shorter interval between initial diagnosis and first
metastasis; and more non-CNS metastases.19 CNS metastases from breast cancer
have also been shown to be relatively radiosensitive9,14 and positive
prognostic factors include stable extracranial disease, a lower recursive
partitioning analysis (RPA) class, a higher Karnofsky Performance Scale score,
fewer brain metastases, a smaller total tumor volume per patient, the presence
of deep cerebral or brainstem metastases, and HER2/neu overexpression. 14
While it has been reported
that men with breast cancer have the same disease-specific and event-free
survival as women, the management of CNS metastases for men still remains
unclear. One report of a male with breast cancer who received WBRT alone for
CNS metastases reported a survival of only 7 weeks after diagnosis, and
advocated the same treatment regimens for male patients as for female patients. 18
Gomez-Raposo et al. 11 reported that the overall 5- and 10-year
survival rates of MBC patients were approximately 60 and 40%,
respectively. Survival time for men with breast cancer was worse than for
women. While Mouna et al. 17 explained that this was due to more
aggressive biologic behavior of male breast cancer, the more commonly used
explanation is that the rarity of male breast cancer makes it difficult to
diagnose at a less advanced stage.
Discuss the most important findings in your study.
In this case report, two of three patients survived more than three years after SRS. One patient with pituitary metastasis died 4 months after SRS due to CNS progression. In our previous report of SRS for pituitary metastases, the median survival was only 5.2 months. 12 Stereotactic radiosurgery was an effective treatment for patients with brain metastases from MBC. Discuss the various aspects of your work (for example, treatment-related complications, comparisons to other approaches or techniques, cost-effectiveness analysis)Radiosurgery has been shown to be a cost-effective approach for the management of brain metastases in comparison to whole brain radiation therapy or surgical resection. 18,21 We did not measure treatment costs in these three patients. Discuss Future Work and Recommendations.
In the future, a case-matched control study between male and female breast cancer brain metastases would be of interest.
Male breast carcinoma (MBC) is extremely uncommon, with incidence of only 1.2 cases per 100,000 men. Management of such rare brain tumors is not well characterized. The purpose of this study was to understand the role of stereotactic radiosurgery (SRS) in the management of brain metastases from MBC. We reviewed records from 3 patients who underwent SRS for the management of their brain metastases, selected from a group of over 4,000 patients with all types of brain metastases managed over a 23 year interval.
In this case report three patients were treated for a total of 4 metastases. Treatment resulted in regression of 2 metastases, no change in 1 metastasis, and progression in 1 metastasis. Median survival time following SRS was 39 months, with a range of 4-47 months. The main limitation of this study was the small sample size. Stereotactic radiosurgery was an effective treatment in patients with brain metastases from male breast cancer. Male patients with breast cancer tend not to do worse than a comparable cohort of female patients treated with SRS. Brain metastases from breast cancer may be managed with SRS similarly in males and females.
Male breast carcinoma (MBC) is extremely uncommon, accounting for less than 1% of all cancer in men. Approximately 2,000 new cases are diagnosed in the United States each year,23 with an incidence of just 1.2 per 100,000 men.12 The etiology of MBC has been linked to BRCA 1 and BRCA 2 mutations, as well as Klinefelter’s syndrome and several acquired and environmental factors.1
Brain metastases develop in 20-40% of patients with cancer, and are a significant cause of morbidity and mortality.14 Due to the rarity of MBC and limited data, clinical management is primarily based on the management of female breast carcinoma (FBC). Treatment modalities for brain metastases from primary breast carcinoma in both males and females include surgical resection, whole brain radiation therapy (WBRT), and more recently, stereotactic radiosurgery (SRS).
There have been no prior reports of the management of CNS metastases from MBC for multiple patients. Therefore, we sought to determine whether SRS was an effective treatment for brain metastases from MBC.
We reviewed three cases of patients with brain metastases from MBC
who were treated with SRS.
Prior reports have suggested that outcomes for males are typically similar to female patients, however there has only been limited data in case report format on male patients with brain metastases. The main limitation of this study was the small sample size.
This retrospective study was approved by the University of Pittsburgh Institutional Review Board. This work was not funded by an external source. At the time of SRS, patient 1 was 51 years old, patient 2 was 67 years old, and patient 3 was 70 years old. Genetic testing was not performed on these patients. All patients were male. Patient 1 was diagnosed with primary MBC 4 years before SRS. At the time, the patient underwent a mastectomy and axillary node dissection. Nearly four years before SRS, the patient was found to have a solitary brain metastasis in the left cerebellar region, which was treated with WBRT. A scan performed one month before SRS revealed progression of the cerebellar tumor along with a small new metastasis in the medial right temporal lobe. At the time of SRS, the patient was neurologically asymptomatic.
Patient 2 was diagnosed with primary MBC 7 years before SRS. The primary site was managed with a right modified radical mastectomy, chemotherapy (Cytoxan and Adriamycin, followed by Tamoxifen then Arimidex), and radiation therapy. He presented to the SRS clinic with right-sided lip and chin numbness and partial diabetes insipidus. Magnetic resonance imaging (MRI) revealed enlargement of the pituitary gland, as well as multiple areas of T2 prolongation throughout the cervical spine and clivus, consistent with metastatic disease from primary breast cancer. There was no noticeable involvement in the cavernous sinus to explain the right V3 numbness. The patient was placed on DDAVP to control the diabetes insipidus, and underwent SRS to the pituitary.
Patient 3 was
diagnosed with primary MBC 8 years before SRS. The primary site was treated with a left modified radical mastectomy, followed by a six-month adjuvant chemotherapy regimen (Cytoxan, Methotrexate, 5-FU, Tamoxifen and Prednisone). The patient was disease-free and asymptomatic until 5 years before SRS, when he began noticing flashing lights in the left visual field. Computed Tomography (CT) imaging revealed a solitary right occipital tumor. This was surgically resected, followed by WBRT (50 Gy in 25 fractions). Post-operatively, his visual field symptoms persisted and the patient developed ataxia. At the time of SRS, MR imaging revealed a small solitary recurrent occipital metastasis.
All patients had physical exams consistent with Karnofsky Performance Status (KPS) >70 at the time of SRS. History of genetic testing for mutations associated with increased risk of breast cancer was unavailable.
Tumor histologic types varied. Patient 1 had adenocarcinoma of unknown stage, histologic grade, or hormone status. Patient 2 was staged T2N1M0, with a histology of infiltrating, moderately differentiated ductal carcinoma, ER+/PR+. Patient 3 was staged
T2N1M0, with a histology of poorly differentiated, infiltrating lobular carcinoma, ER+/PR+, with tumor present in four axillary lymph nodes.
Gamma knife stereotactic radiosurgery was performed in all three patients (Elekta, Norcross GA). Patient 1 received 15 Gy to the margin of his cerebellar tumor, which measured 2.9 cc (maximum dose 30 Gy) and 15 Gy to the margin of his temporal lobe tumor, which measured 1.9cc (maximum dose 21.43 Gy). Patient 2 received 16 Gy to the margin of a 1.3cc tumor in the clivus (maximum dose, 32 Gy) (Figure 1). The maximum dose to the optic nerve was 8 Gy. Patient 3 received 20 Gy to the margin of his 0.3cc occipital tumor (maximum dose 40 Gy). No patient underwent additional whole brain radiotherapy or surgical resection after receiving SRS.
Changes in tumor size were determined by contrast-enhanced MR imaging for all patients, and symptomatic changes were determined by clinical correspondence. All subjects had both clinical and imaging followup after SRS. The follow-up assessments were evaluated by clinicians not involved directly in patient care. The outcomes measures were validated for this study. No statistics were calculated, as only three patients were in the study. This study did not include a power calculation. Due to the small sample size, no statistical comparisons were performed. Data Share 1: Patient Characteristics with Breast Cancer| # of patients (Count) | age - median years | sex-male (Count) | Metastases-breast cancer (Count) | Follow-up time (months) (Avg) | prior resection (Count) | prior radiotherapy (Count) |
|---|
| 3 | 67 | 3 | 4 | 14.4 | 1 | 2 |
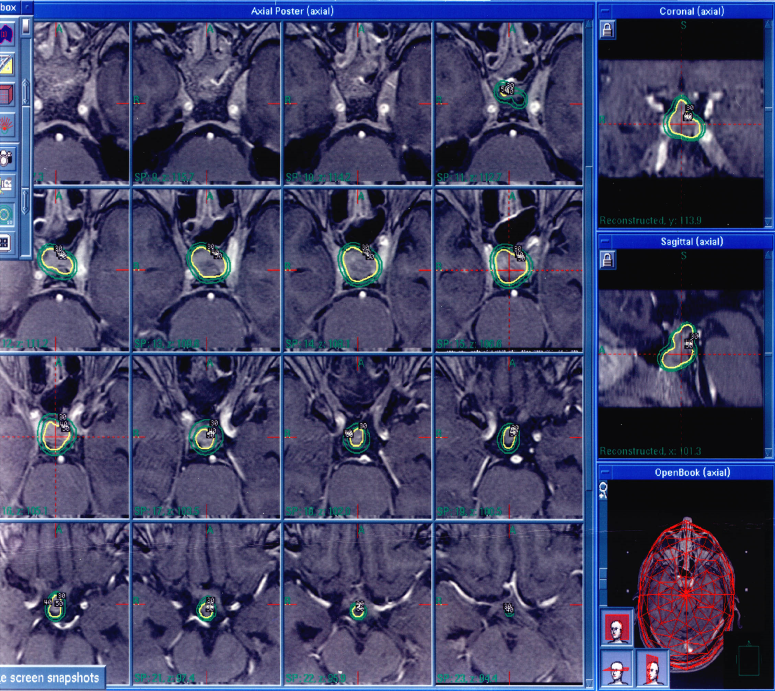 |
| Consecutive axial MR images,T1-weighted with contrast, of Patient 2 performed preoperatively. Gamma knife planning software was used to target the patient's pituitary lesion, circled above in green and yellow. |
We followed patients serially with imaging and clinical assessments. Overall patient survival is shown in Data Share 2. The survival of this small series varied widely. Patient 1 died 39 months after SRS due to CNS progression. Patient 2 died 4 months after SRS, possibly due to CNS progression. Patient 3 died 47 months later due to unknown causes. Clinical Response
The clinical response to radiosurgery is shown in Data Shares 3-5. In this report, we analyzed the response to symptoms present at the time of SRS. Patient 1, who initially presented without symptoms, began to experience short-term memory loss over the course of 2-3 years following SRS, likely due to progression of the tumor in the right temporal lobe. Patient 2, who presented with facial numbness in the V3 region and partial diabetes insipidus, experienced increased numbness one month after SRS. The numbness resolved within one week after administration of corticosteroids to control edema. The patient also developed full central diabetes insipidus, which was controlled with continued oral DDAVP. Patient 3, who initially presented with ataxia and a left visual field cut, initially experienced symptom resolution 6 months following SRS and became completely asymptomatic 10 months following SRS.
Imaging Response
The imaging response to radiosurgery is shown in Data Shares 6-8. Patient 1, who received SRS for both a right temporal tumor and a left cerebellar tumor, showed regression of both tumors for the first 22 months after SRS. Imaging performed 30 months after SRS showed almost no residual tumor in the cerebellum, but significant enlargement of the right temporal tumor, which had a diameter increase from 1.3cc at 22 months to 3.0cc at 30 months. The patient received no treatment for the right temporal progression. Patient 2, who received treatment for a pituitary metastasis, showed a stable tumor one month following SRS (Figure 2). However, he had a new tumor in the choroid plexus. The patient was subsequently lost to follow-up and received no treatment for the new metastasis. Patient 3, who received treatment for a small solitary right occipital metastasis, showed tumor regression 3 months following SRS, followed by complete disappearance 10 months after SRS.
Because of the small sample size, multivariate testing was not performed. Data Share 2: Summary of Outcomes after Brain Metastasis RadiosurgeryTotal number of metastases treated, final treatment outcome, and average patient survival.| # of patients (Count) | Metastases (Count) | Tumor response-larger (Count) | Tumor response-smaller (Count) | Tumor response-unchanged (Count) | Survival months (Avg) |
|---|
| 3 | 4 | 1 | 2 | 1 | 30 |
| prior resection | prior radiotherapy | RPA Class (metastases) | Radiation Dose (mean; Gy) | symptom | Outcome | Follow-up time (months) |
|---|
| No | No | 2 | 16 | Right V3 Numbness, partial diabetes insipidus | Increased V3 Numbness | 1.0 |
| prior resection | prior radiotherapy | RPA Class (metastases) | Radiation Dose (mean; Gy) | symptom | Outcome | Follow-up time (months) |
|---|
| No | Yes | 1 | 15 | No Symptoms | Short-term memory loss | 31.2 |
| prior resection | prior radiotherapy | RPA Class (metastases) | Radiation Dose (mean; Gy) | symptom | Outcome | Follow-up time (months) |
|---|
| Yes | Yes | 2 | 20 | Left VFD, ataxia | Resolution of Symptoms | 10.9 |
| Metastases (Count) | location | Tumor volume ml (Sum) | Tumor volume ml | Imaging Response | Outcome | Follow-up time (months) |
|---|
| 1 | Pituitary | 1.3 | 1.3 | Stable tumor, new met in the choroid plexus | No further treatment | 1.0 |
| Metastases (Count) | location | Tumor volume ml (Sum) | Tumor volume ml | Imaging Response | Outcome | Follow-up time (months) |
|---|
| 2 | Left Cerebellum, Right Temporal | 4.8 | 2.9 | Left cerebellar regression, Right temporal progression | No further treatment | 31.2 |
| Metastases (Count) | location | Tumor volume ml (Sum) | Tumor volume ml | Imaging Response | Outcome | Follow-up time (months) |
|---|
| 1 | Right Occipital | 0.3 | 0.3 | Regression | No further treatment | 10.9 |
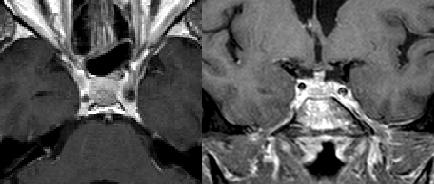 |
| Axial and coronal MRI, T1-weighted with contrast, of Patient 2's pituitary lesion. Performed one month post-SRS, this image demonstrates a regressed pituitary metastasis. |
CNS involvement due to male
breast cancer drastically affects both the treatment options and prognosis for
patients.4,6,8,15 There are several notable differences between MBC
and FBC. For example, MBCs are mostly ductal in origin,1,20 and 90.6% of MBCs are ER+, compared to 76.0% of FBCs.
Similarly, 81.2% of MBCs are PR+ compared to 66.7% of FBCs.1,10
HER-1 positivity has been reported as ranging from as low as 1.1%3
to as high as 37% in men,10 compared to approximately 26-27% in
women.1,3 Arslan et al. 2 reported in their non-metastatic male breast cancer series
(N=118), the proportions of positivity of estrogen receptor (ER),
progesterone receptor (PGR), and HER2 status were 82.9, 75.8, and 23.4%,
respectively. In our previous FBC SRS series (N=350), the proportion of
positivity of ER and HER2 status were 50 and 59%, respectively.14 Additionally, MBCs present an average of 10 years after
FBCs.1,5,7,13
All studies that have
considered the management of CNS metastases from breast cancer consisted of
entirely female or combined male and female populations. It was shown that the
risk of developing brain metastasis from breast cancer in a combined male and
female population is increased in patients with the following characteristics:
a younger age; a higher histological grade; estrogen receptor positive,
progesterone receptor positive, and HER2 negative > triple negative status
> HER2 positive; a shorter interval between initial diagnosis and first
metastasis; and more non-CNS metastases.19 CNS metastases from breast cancer
have also been shown to be relatively radiosensitive9,14 and positive
prognostic factors include stable extracranial disease, a lower recursive
partitioning analysis (RPA) class, a higher Karnofsky Performance Scale score,
fewer brain metastases, a smaller total tumor volume per patient, the presence
of deep cerebral or brainstem metastases, and HER2/neu overexpression.14
While it has been reported
that men with breast cancer have the same disease-specific and event-free
survival as women, the management of CNS metastases for men still remains
unclear. One report of a male with breast cancer who received WBRT alone for
CNS metastases reported a survival of only 7 weeks after diagnosis, and
advocated the same treatment regimens for male patients as for female patients.18
Gomez-Raposo et al. 11 reported that the overall 5- and 10-year
survival rates of MBC patients were approximately 60 and 40%,
respectively. Survival time for men with breast cancer was worse than for
women. While Mouna et al. 17 explained that this was due to more
aggressive biologic behavior of male breast cancer, the more commonly used
explanation is that the rarity of male breast cancer makes it difficult to
diagnose at a less advanced stage.
In this case report, two of three patients survived more than three years after SRS. One patient with pituitary metastasis died 4 months after SRS due to CNS progression. In our previous report of SRS for pituitary metastases, the median survival was only 5.2 months. 12 Stereotactic radiosurgery was an effective treatment for patients with brain metastases from MBC. Radiosurgery has been shown to be a cost-effective approach for the management of brain metastases in comparison to whole brain radiation therapy or surgical resection.18,21 We did not measure treatment costs in these three patients.
In the future, a case-matched control study between male and female breast cancer brain metastases would be of interest.
The Author(s) wish to thank:Project Roles:
1. Agrawal, A.,Ayantunde, AA.,Rampaul, R.,Robertson, JF., Male breast cancer: a review of clinical management. Breast Cancer Res Treat 103: 11 - 21, 20072. Arslan, UY.,Oksuzoglu, B.,Ozdemir, N.,Aksoy, S.,Alkis, N.,Gok, A., et al: Outcome of non-metastatic male breast cancer: 118 patients. Med Oncol : - , 20113. Bloom, KJ.,Govil, H.,Gattuso, P.,Reddy, V.,Francescatti, D., Status of HER-2 in male and female breast carcinoma. Am J Surg 182: 389 - 392, 20014. Boogerd, W.,Vos, VW.,Hart, AA.,Baris, G., Brain metastases in breast cancer; natural history, prognostic factors, and outcome. J Neurooncol 15: 165 - 174, 19935. Borgen, PI.,Wong, GY.,Vlamis, V.,Potter, C.,Hoffmann, B.,Kinne, DW., et al: Current management of male breast cancer. A review of 104 cases. Ann Surg 215: 451 - 457; discussion 457-459, 19926. Dawood, S.,Broglio, K.,Esteva, FJ.,Ibrahim, NK.,Kau, SW.,Islam, R., et al: Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol 19: 1242 - 1248, 20087. de Perrot, M.,Deleaval, J.,Robert, J.,Spiliopoulos, A., Thirty-year experience of surgery for breast carcinoma in men. Eur J Surg 166: 929 - 931, 20008. DiLuna, ML.,King, JT.,Knisely, JP Jr.,Chiang, VL., Prognostic factors for survival after stereotactic radiosurgery vary with the number of cerebral metastases. Cancer 109: 135 - 145, 20079. Firlik, KS.,Kondziolka, D.,Flickinger, JC.,Lunsford, LD., Stereotactic radiosurgery for brain metastases from breast cancer. Ann Surg Oncol 7: 333 - 338, 200010. Giordano, SH.,Buzdar, AU.,Hortobagyi, GN., Breast cancer in men. Ann Intern Med 137: 678 - 687, 200211. Gomez-Raposo, C.,Zambrana Tevar, F.,Sereno Moyano, M.,Lopez Gomez, M.,Casado, E., Male breast cancer. Cancer Treat Rev 36: 451 - 457, 201012. Howlader, N.,Noone, AM.,Krapcho, M.,Neyman, N.,Aminou, R.,Waldron, W., SEER Cancer Statistics Review, 1975-2008. Based on November 2010 SEER data submission, posted to the SEER web site, 2011. National Cancer Institude. 10-26-2011. < http://seer.cancer.gov/csr/1975_2008/ >.13. Kano, H.,Niranjan, A.,Kondziolka, D.,Flickinger, JC.,Lunsford, LD., Stereotactic radiosurgery for pituitary metastases. Surg Neurol 72: 248 - 255; discussion 255-246, 200914. Koc, M.,Polat, P., Epidemiology and aetiological factors of male breast cancer: a ten years retrospective study in eastern Turkey. Eur J Cancer Prev 10: 531 - 534, 200115. Kondziolka, D.,Kano, H.,Harrison, GL.,Yang, HC.,Liew, DN.,Niranjan, A., et al: Stereotactic radiosurgery as primary and salvage treatment for brain metastases from breast cancer. J Neurosurg 114: 792 - 800, 201116. Lin, NU.,Bellon, JR.,Winer, EP., CNS metastases in breast cancer. J Clin Oncol 22: 3608 - 3617, 200417. Marchal, F.,Salou, M.,Marchal, C.,Lesur, A.,Desandes, E., Men with breast cancer have same disease-specific and event-free survival as women. Ann Surg Oncol 16: 972 - 978, 200918. Mehta, M.,Noyes, W.,Craig, B.,Lamond, J.,Auchter, R.,French, M., A cost-effectiveness and cost-utility analysis of radiosurgery vs. resection for single-brain metastases . International Journal of Radiation Oncology Biology Physics 39(2): 445 - 454, 199719. Mouna, B.,Rhizlane, B.,Amine, S.,Hind, M.,Fouad, T.,Hassan, E., Male breast cancer: a report of 127 cases at a Moroccan institution. BMC Res Notes 4: 219 - , 201120. Nieder, C.,Jost, PJ.,Grosu, AL.,Peschel, C.,Molls, M., Report of a male patient with brain metastases from breast cancer. Breast 12: 345 - 347, 200321. Rutigliano, MJ.,Lunsford, LD.,Kondziolka, D.,Strauss, MJ.,Khanna, V.,Green, M., The cost effectiveness of stereotactic radiosurgery versus surgical resection in the treatment of solitary metastatic brain tumors. Neurosurgery 37(3): 445 - 455, 199522. Sheehan, J., Radiosurgery for breast cancer. J Neurosurg 114: 790 - 791; discussion 791, 201123. Siegel, R.,Ward, E.,Brawley, O.,Jemal, A., Cancer statistics, 2011. CA: A Cancer Journal for Clinicians 61: 212 - 236, 201124. Stalsberg, H.,Thomas, DB.,Rosenblatt, KA.,Jimenez, LM.,McTiernan, A.,Stemhagen, A., et al: Histologic types and hormone receptors in breast cancer in men: a population-based study in 282 United States men. Cancer Causes Control 4: 143 - 151, 1993
|

