Pulse Generator Corrosion as a Complication of Vagus Nerve Stimulator ImplantationKeywords: vagus nerve stimulation, deep brain stimulation, epilepsy, complications, Inflammatory MassesInteractive ManuscriptAsk Questions of this Manuscript: What is the background behind this topic?Vagal nerve stimulation (VNS) is a common procedure for medically-refractory complex epilepsy. Hardware-related complications typically consist of infection or fracture of the electrode. Non-infectious morbidity related to the pulse generator is rare. Describe your observation(s).We cared for a 27 year old female referred to us 15 months after VNS placement at another hospital. She developed an expanding mass over the pulse generator. Surgical exploration revealed a 2 cm thick wall of granulation tissue completely surrounding the pulse generator, which was found to be corroded at the electrode-generator connection. Generator power had become depleted early. Describe the major lesson provided by your observation.Breakage or an open circuit at the electrode-generator interface can lead to early loss of power and a marked inflammatory reaction surrounding the generator. This will appear as an expanding mass over the pulse generator. What is the background of your topic ?Implantation of pulse generators for the care of patients with pain, epilepsy, movement and behavioral disorders, and cardiac dysrhythmias are becoming increasingly common. Complications related to hardware placement can cause morbidity and malfunction of the device. The identification of these complications can be made from clinical examination, needle aspiration of fluid-filled pockets, or device inspection and removal. The commonest problem associated with pulse generator placement is local site infection (1,2,3,5,6,8,9,12). Complication profiles related to deep brain stimulators, vagus nerve stimulators, and cardiac pacemakers have been reported (4,7,10,11), but no reports of overt corrosion have been made. However, other sterile tissue responses can occur. This patient did not have any predisposition for granuloma formation or other tissue responses such as keloids. Describe your main observation and why it is important.We managed a patient with a presumed infected pulse generator pocket who had surgery at another institution. Two needle aspirates of fluid were sterile, with reaccumulation of the mass. The mass was approximately 3 cm above the skin surface, and was mildly tender. The skin was not erythematous. There was no fluid along the VNS cable and no sign of inflammation or fluid in the neck. An x-ray showed that the system appeared intact. Blood serology was normal. The patient had sustained clinical benefit from VNS therapy and had no seizures in the six months prior to battery removal.
Because no cause of the chest wall mass had been identified, we recommended surgical exploration. At surgery, we found a 2 cm thick wall of granulation tissue that covered the generator completely on all sides. Within the lesion was brownish tissue fluid. The pulse generator was removed and at the electrode-generator block, we found a hard blackened lesion that was cut from the block using a knife. The VNS electrode lead was soft and broke in our fingers. Thus, the lead was cut as it exited the exposure just below the level of the clavicle. Because the patient remained seizure free, a new system has not been implanted now after one year. Has this observation been reported previously?Although there have been cases which describe rapid decline in battery longevity from current leaks associated with lead fracture (11), there has not been a previous report of an implanted battery becoming corroded (VNS or other type) causing a recurrent fluid collection requiring surgical removal. Pathologic evaluation showed corrosion and breakage of the electrode at its connection to the pulse generator. The patient's family obtained the device for an independent analysis which has not yet been reported. If your work has Institutional Review Board or other supervisory authority approval, state that now:This report was authorized by the University of Pittsburgh Institutional Review Board. State the source of funding for this study.This study was not funded by any external source. Describe how your observation was made (ie. specific tests, other)The observations in this report were made from clinical evaluation, needle aspiration of the chest wall inflammatory mass and culture studies, surgical removal and histological evaluation of the pulse-generator pocket, and visual inspection of the corroded pulse generator. The pathology showed intense chronic inflammatory reaction surrounding the generator. The gross appearance of the generator showed blackened staining. The electrode lead pin was soft and easily broke with limited manipulation. Describe the most important lesson(s) of this report.The most important lesson of this report is that battery corrosion can lead to a slowly expanding mass over the pulse generator site, and likely associated with rapid decay in battery power. The clinical appearance can mimic an infection except the patient does not appear sick and the signs of inflammation are unlikely to track along the cable. We believe that the corrosion was due to leakage of battery chemicals, although this remains unknown. The description of the device by the manufacturer notes possible "corrosion of the stimulating lead" (http://www.fda.gov/ohrms/dockets/ac/04/briefing/4047b1_02_Summary%20of%20Safety%20and%20Effectiveness.pdf)
Now one year after surgery, the patient has remained well with no further tissue reaction. She has not requested replacement of the VNS system. Describe the limitations of your findings.Because this report includes only the observation from one case, it is possible that other clinical manifestations can be related to pulse generator corrosion in addition to what we have described. Discuss the relevance of your observation to the reader.The relevance of this observation is that a sterile fluid-filled mass surrounding an implanted pulse generator may be due to a damaged or broken connection between the cable and generator leading to corrosion. This observation was reported to the manufacturer. However, the device was sent by the patient for an independent evaluation. The manufacturer was requested to report this incident to the United States Food and Drug Administration. Vagal nerve stimulation (VNS) is a common procedure for medically-refractory complex epilepsy. Hardware-related complications typically consist of infection or fracture of the electrode. Non-infectious morbidity related to the pulse generator is rare. We cared for a 27 year old female referred to us 15 months after VNS placement at another hospital. She developed an expanding mass over the pulse generator. Surgical exploration revealed a 2 cm thick wall of granulation tissue completely surrounding the pulse generator, which was found to be corroded at the electrode-generator connection. Generator power had become depleted early. Breakage or an open circuit at the electrode-generator interface can lead to early loss of power and a marked inflammatory reaction surrounding the generator. This will appear as an expanding mass over the pulse generator. Implantation of pulse generators for the care of patients with pain, epilepsy, movement and behavioral disorders, and cardiac dysrhythmias are becoming increasingly common. Complications related to hardware placement can cause morbidity and malfunction of the device. The identification of these complications can be made from clinical examination, needle aspiration of fluid-filled pockets, or device inspection and removal. The commonest problem associated with pulse generator placement is local site infection (1,2,3,5,6,8,9,12). Complication profiles related to deep brain stimulators, vagus nerve stimulators, and cardiac pacemakers have been reported (4,7,10,11), but no reports of overt corrosion have been made. However, other sterile tissue responses can occur. This patient did not have any predisposition for granuloma formation or other tissue responses such as keloids.
We managed a patient with a presumed infected pulse generator pocket who had surgery at another institution. Two needle aspirates of fluid were sterile, with reaccumulation of the mass. The mass was approximately 3 cm above the skin surface, and was mildly tender. The skin was not erythematous. There was no fluid along the VNS cable and no sign of inflammation or fluid in the neck. An x-ray showed that the system appeared intact. Blood serology was normal. The patient had sustained clinical benefit from VNS therapy and had no seizures in the six months prior to battery removal.
Because no cause of the chest wall mass had been identified, we recommended surgical exploration. At surgery, we found a 2 cm thick wall of granulation tissue that covered the generator completely on all sides. Within the lesion was brownish tissue fluid. The pulse generator was removed and at the electrode-generator block, we found a hard blackened lesion that was cut from the block using a knife. The VNS electrode lead was soft and broke in our fingers. Thus, the lead was cut as it exited the exposure just below the level of the clavicle. Because the patient remained seizure free, a new system has not been implanted now after one year.
Although there have been cases which describe rapid decline in battery longevity from current leaks associated with lead fracture (11), there has not been a previous report of an implanted battery becoming corroded (VNS or other type) causing a recurrent fluid collection requiring surgical removal. Pathologic evaluation showed corrosion and breakage of the electrode at its connection to the pulse generator. The patient's family obtained the device for an independent analysis which has not yet been reported.
Figure 1:
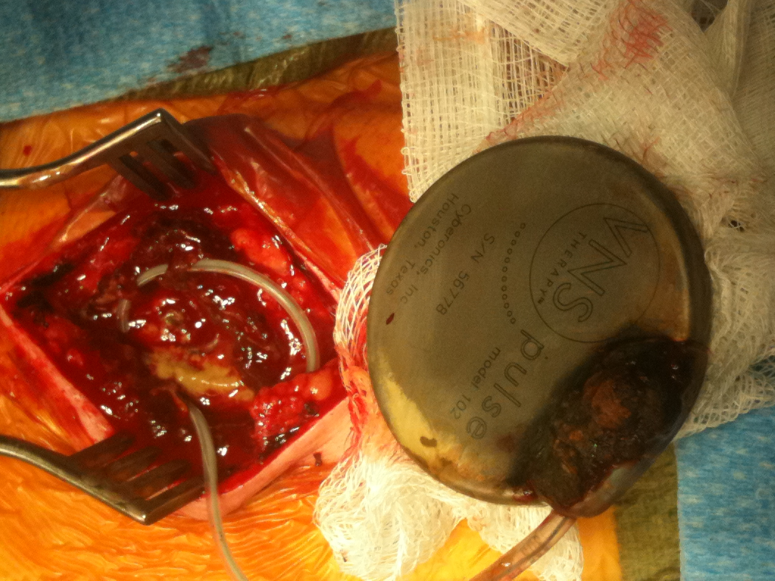 |
| Vagal nerve stimulator pulse generator showing corrosion at the cable-generator interface |
This report was authorized by the University of Pittsburgh Institutional Review Board. This study was not funded by any external source. The observations in this report were made from clinical evaluation, needle aspiration of the chest wall inflammatory mass and culture studies, surgical removal and histological evaluation of the pulse-generator pocket, and visual inspection of the corroded pulse generator. The pathology showed intense chronic inflammatory reaction surrounding the generator. The gross appearance of the generator showed blackened staining. The electrode lead pin was soft and easily broke with limited manipulation.
The most important lesson of this report is that battery corrosion can lead to a slowly expanding mass over the pulse generator site, and likely associated with rapid decay in battery power. The clinical appearance can mimic an infection except the patient does not appear sick and the signs of inflammation are unlikely to track along the cable. We believe that the corrosion was due to leakage of battery chemicals, although this remains unknown. The description of the device by the manufacturer notes possible "corrosion of the stimulating lead" (http://www.fda.gov/ohrms/dockets/ac/04/briefing/4047b1_02_Summary%20of%20Safety%20and%20Effectiveness.pdf)
Now one year after surgery, the patient has remained well with no further tissue reaction. She has not requested replacement of the VNS system.
Because this report includes only the observation from one case, it is possible that other clinical manifestations can be related to pulse generator corrosion in addition to what we have described. The relevance of this observation is that a sterile fluid-filled mass surrounding an implanted pulse generator may be due to a damaged or broken connection between the cable and generator leading to corrosion. This observation was reported to the manufacturer. However, the device was sent by the patient for an independent evaluation. The manufacturer was requested to report this incident to the United States Food and Drug Administration.
The Author(s) wish to thank:Project Roles:
1. Chan, DT.,Zhu, XL.,Yeung, JH.,Mok, VC.,Wong, E.,Lau , C., et al: Complications of deep brain stimulation: a collective review. Asian J Surg 32: 258 - 263, 20092. Constantoyannis , C.,Berk, C.,Honey, CR.,Mendez, I.,Brownstone, RM., Reducing hardware-related complications of deep brain stimulation. Can J Neurol Sci 32: 194 - 200, 20053. Farris, S.,Vitek, J.,Giroux , ML., Deep brain stimulation hardware complications: the role of electrode impedance and current measurements. Mov Disorders 23: 755 - 760, 20084. Kabir, SM.,Rajaraman, C.,Rittey, C.,Zaki, HS.,Kemeny, AA.,McMullan, J., Vagus nerve stimulation in children with intractable epilepsy: indications, complications and outcome. Childs Nerv System 25: 1097 - 1100, 20095. Kondziolka, D.,Whiting, D.,Germanwala, A.,Oh, M., Hardware-related complications from thalamic deep brain stimulator systems. Stereotact Funct Neurosurg 79: 228 - 233, 20026. Oh, MY.,Abosch, A.,Kim, SH.,Lang , AE.,Lozano, AM., Long-term hardware-related complications of deep brain stimulation. Neurosurgery 50: 1268 - 1274, 20027. Oto, A.,Aytemir, K.,Yorgun , H.,Canpolat , U.,Kaya , EB.,Kabak?i , G., et al: Percutaneous extraction of cardiac pacemaker and implantable cardioverter defibrillator leads with evolution mechanical dilator sheath: a single-centre experience.. Europace 13: 543 - 547, 20108. Paluzzi, A.,Bell, A.,Bain , P.,Liu, X.,Aziz, TM., Operative and hardware complications of deep brain stimulation for movement disorders. Br J Neurosurg 20: 290 - 295, 20069. Sillay, KA.,Larson , PS.,Starr, PA., Deep brain stimulator hardware-related infections: incidence and management in a large series. Neurosurgery 62: 360 - 366, 200810. Spuck , S.,Tronnier, V.,Orosz, I.,Schonweiler, R.,Sepehrnia, A.,Nowak, G., et al: Operative and technical complications of vagus nerve stimulator implantation. Neurosurgery 67: 489 - 494, 201011. van Gelder, LM.,El Gamal , MI., False inhibition of an atrial demand pacemaker caused by an insulation defect in a polyurethane lead. Pacing Clin Electrophsyiol 6: 834 - 839, 198312. Voges, J.,Waerzeggers, Y.,Maarouf, M.,Lehrke, R.,Koulousakis, A.,Lenartz, D., et al: Deep-brain stimulation: long-term analysis of complications caused by hardware and surgery--experiences from a single centre. J Neurol Neurosurg Psych 77: 868 - 872, 2006
|

