A ventricle staining technique for formalized cadaver brain for studies in neurosurgical anatomyKeywords: education, cadaver dissection, surgical anatomy, staining technique, VentricleInteractive ManuscriptAsk Questions of this Manuscript: What is the background behind your study?The transcortical approach to the ventricular system can be practiced in the formalin preserved cadaver brain. Although there are many advantages using a specimen of this nature, there are technical difficulties that a dissector may encounter during the approach to the temporal horn.
What is the purpose of your study?Here we describe a staining technique that we use in our center. This will help the dissector in the approach to the temporal horn. It can also be used in photographic documentation of the same area for descriptive anatomy.
Describe your primary experiment.Six cadaver brains were used. This means twelve temporal lobes were available for evaluation. Three investigators participated. Each had to dissect two non-stained and two stained temporal lobes. Describe any additional experiments.This question was not answered by the author Describe your main findings. This technique stained the ventricular cavity well enough to help the trainees in their initial learning process. Describe the main limitation of this study.However, if there is an unforeseen obstruction within the ventriclular cavity due to a tumor or some adhesion, the stain may not travel far enough to produce the expected result. Another drawback is when the specimen is retransferred to and from the formalin bath several times in between dissections, a significant portion of the stain is lost within a period of four weeks. Describe your main conclusion.This question was not answered by the author Describe the importance of your findings and how they can be used by others.Techniques of this kind will help the neurosurgery trainees during their initial phase of learning. Other centers could adopt and develop this technique further to suit the neurosurgery practise sessions. What is the background of your topic? To master the transcortical approaches to the intraventricular system, various types of specimens can be used  . In our center we use formalized human cadaver brain. The main advantage of using the human specimen is that one will appreciate the exact anatomy  . In our center cadaver brains are freely available due to frequent donations for teaching purposes. Thus, the cost for this tissue is low. What is the importance to the reader/community? In our program the trainee is expected to do a descriptive anatomy dissection of the part in attention, prior to the micro-dissection practice sessions. This is because in a narrow window of a micro dissection it is difficult to appreciate the important anatomic details of the adjacent region. One should master a region as a whole and not confine his knowledge to the area of microdissection. A gross dissection prior to micro dissection will complete this task. Therefore, the dissector is expected to approach the chosen area with a naked eye or loupe vision to appreciate the gross architecture at the onset itself. What is your hypothesis? (What question(s) did you ask?) But, there are a few drawbacks in using a formalized specimen. This is especially seen in the cortical approaches to the temporal horn. Macroscopic identification of the temporal horn in the preserved cadaver is not that easy. It is almost always collapsed. In contrast, the cavity of the body of the lateral ventricle is kept open due to the firmness of the nearby structures. What was done in your study? Consistency of the formalin fixed cadaver brain is not the same as the living brain that is encountered in surgery. While the living brain is pliable and can be retracted, the formalized cadaver brain will start to crack during retraction. The parenchyma of living brain could be dissected with the knife or the CUSA the way the operator wishes. But, the formalized cadaver brain will break into strips and small chunks even to the strokes of the sharpest knife. Also, in the living brain one might see emission of CSF as soon as the temporal horn ependyma is breached; the choroid too, might peek through. The cadaver brain ventricle will not show CSF emission and the choroid is plastered to the inner surface of the ventricle. So an inexperienced trainee might enter the temporal horn and will go through to the opposite wall even without noticing. This is true whether the ventricle was reached with the naked eye vision or with the loupes. Even with the dissecting microscope there is a chance of missing the ventricle due to a difficult retraction. Therefore, a stained ventricle wall will no doubt help the dissector confirming that he has entered the ventricle. Thus, in our center we developed a technique to stain the ventricles. What are the main conclusions derived from prior reports?We also use the same technique when a brain specimen is dissected and photographed for teaching purposes. What are the main limitations of your research method?In our series all specimens stained as expected. However, if there is an unforeseen obstruction within the ventriclular cavity due to a tumor or some adhesion, the stain may not travel far enough to produce the expected result. Another drawback is when the specimen is retransferred to and from the formalin bath several times in between dissections, a significant portion of the stain is lost over the first month. If your work has Research Committee (ie. Animal care) approval, state that now:This study was approved by the medical school research committee. State the source of funding for this study.The specimens were donated by citizens supporting medical research. Therefore no external funding was involved. Describe the design of your primary experiment. The items needed are, a formalized cadaver brain with an intact pia (harvested out of the cranium), a bottle of red ink (fountain pen ink), a 20cc syringe and an 18 gauge needle.
On the basal surface of the brain one can visualize the circle of Willis, with the open ends of the internal carotid and vertebral/basilar arteries. Veins are collapsed in most of the specimens, but could be identified beneath the arachnoid. Pure red ink is injected with some force to the open ends of each artery. Sometimes intra-arterial clots will limit the efficiency of this process. One could see that the ink start to leak from the unidentified torn vascular openings and will start to stain the basal surface of the brain. This may even alarm the dissector with the thought that the specimen will over stain. If this happens hold the entire brain under running water to remove excess ink.
Following this, the user should inject a few milliliters of ink into the 3rd ventricle through its floor. The brain should be kept upside down so that the ink will travel well to the lateral ventricles. Then rock the brain sideways for 4 to 5 minutes for the ink to spread uniformly inside the lateral ventricles. Now turn the brain specimen to its correct orientation. Then the excess red ink will dribble from the 3rd ventricular opening and from the 4th ventricle. Immediately transfer the specimen to the sink and leave it under running water to take away the excess stain. This is important as at this stage any excess stain will turn the brain surface to a red globe. This is because excess ink will seep under the breeched pia. Allow the excess water to drip into the sink. The specimen is then transferred to the usual 10% formalin container. Leave it there for 24 hours. After 24 hours take the specimen out of the bath and allow the excess formalin to drip away. If the formalin vapor is too strong use running water for 2-3 minutes to neutralize the effect. Now the brain specimen is ready for dissection. Describe any important features of your experimental or control groups The dissector will notice that although the brain surface has turned pink (and not red), it is the pia which is stained. Carefully remove the pia in the area that is subjected to dissection. Then the natural dusky-white color of the cadaver brain will reappear. Now the dissector can start the dissection. Describe the techniques used in your primary experiment. Specimen is dissected starting from the outer surface and working towards the ventricles. As soon as the dissection enters the ventricle a dark pink color is noted. The dissector will appreciate that most of the time the ventricle is collapsed and without the stain he would have easily gone through the ventricle and even cut through the opposite wall. He will also notice that the choroid plexus is collapsed and pasted to the ventricular surface, but has a glowing pinkish granular appearance. Choroid is not well seen in the non-stained specimen. Beyond this point the dissector will need the dissecting microscope. If the specimen is used for photo documentation the lateral wall is opened widely to get a better view. Describe any additional experiments and the techniques used.This question was not answered by the author Describe the tests used to perform your research (assays, measurements, other)This question was not answered by the author Describe who conducted the tests (study investigators or other parties; were they experienced in the use of these measures?) Three investigators independantly evaluated the results. Six brain specimens were used (twleve temporal horns). Three brains were stained and three were not. Each investigator had to approach the temporal horn of a non-stained specimen followed by an approach to the temporal horn of a stained specimen. Then the sequence was repeated once more on fresh specimens. One dissector was able to identify the 2nd non-stained temporal horn without much difficulty. Even then, at the ventricular entry point he cut through the unstained choroid that was plastered to the lateral wall. Were the tests validated for use in this kind of study?This form of testing was not validated and was purely observational. Describe your statistical methods or tests usedNo statistics were pertinent to this study. Describe your study power calculation (if any)This question was not answered by the author Describe your chosen level of statistical significanceThis question was not answered by the author Provide the results for the most important outcome of your research. The photographs displayed here are selected from a series of an approach to temporal horn. In these specimens a wider window is made for the purpose of clear demonstration; usually a dissector uses a smaller window. There is a difference in color in side ventricle, parenchyma and the brain stem   . The arteries near the brain stem are visualized in a glistening purple (when a thrombus is inside) and glistening red (when the lumen is empty) 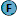 . Discuss any additional findings from your research.This question was not answered by the author Discuss the statistical analyses.This question was not answered by the author Provide the background and reason for your work and briefly summarize important prior research.Many approaches are used to access the ventricular system to treat various pathological conditions     . Hence, mastering the techniques to enter the ventricular system are an important aspect of neurosurgical training. Brain specimens from human cadavers or from other animals are used for this purpose  . Discuss the most important findings in your study.In this laboratory report we are recording a technique that is used in our center for ventricular staining of formalized cadaver brain. It is simple, inexpensive and easy to perform. The results are consistent and meaningful to the trainee. Discuss Future Work and Recommendations.In our center we use 10% formalin as a preservative and keep the specimen in the solution for 2 months. We do not use the freezing method used in other centers. Then we subject these cadaver specimens to the staining method described above.
The staining technique described here will highlight the ventricular system. It should help the dissector who is practicing a cortical approach to the ventricles. This is especially true when a dissector starts his training session with a naked eye or loupe vision. It could also be used to demonstrate the intraventricular contents in photo recording in descriptive brain anatomy. The transcortical approach to the ventricular system can be practiced in the formalin preserved cadaver brain. Although there are many advantages using a specimen of this nature, there are technical difficulties that a dissector may encounter during the approach to the temporal horn.
Here we describe a staining technique that we use in our center. This will help the dissector in the approach to the temporal horn. It can also be used in photographic documentation of the same area for descriptive anatomy.
Six cadaver brains were used. This means twelve temporal lobes were available for evaluation. Three investigators participated. Each had to dissect two non-stained and two stained temporal lobes. This technique stained the ventricular cavity well enough to help the trainees in their initial learning process. However, if there is an unforeseen obstruction within the ventriclular cavity due to a tumor or some adhesion, the stain may not travel far enough to produce the expected result. Another drawback is when the specimen is retransferred to and from the formalin bath several times in between dissections, a significant portion of the stain is lost within a period of four weeks. Techniques of this kind will help the neurosurgery trainees during their initial phase of learning. Other centers could adopt and develop this technique further to suit the neurosurgery practise sessions. To master the transcortical approaches to the intraventricular system, various types of specimens can be used9 . In our center we use formalized human cadaver brain. The main advantage of using the human specimen is that one will appreciate the exact anatomy6 . In our center cadaver brains are freely available due to frequent donations for teaching purposes. Thus, the cost for this tissue is low. In our program the trainee is expected to do a descriptive anatomy dissection of the part in attention, prior to the micro-dissection practice sessions. This is because in a narrow window of a micro dissection it is difficult to appreciate the important anatomic details of the adjacent region. One should master a region as a whole and not confine his knowledge to the area of microdissection. A gross dissection prior to micro dissection will complete this task. Therefore, the dissector is expected to approach the chosen area with a naked eye or loupe vision to appreciate the gross architecture at the onset itself. But, there are a few drawbacks in using a formalized specimen. This is especially seen in the cortical approaches to the temporal horn. Macroscopic identification of the temporal horn in the preserved cadaver is not that easy. It is almost always collapsed. In contrast, the cavity of the body of the lateral ventricle is kept open due to the firmness of the nearby structures. Consistency of the formalin fixed cadaver brain is not the same as the living brain that is encountered in surgery. While the living brain is pliable and can be retracted, the formalized cadaver brain will start to crack during retraction. The parenchyma of living brain could be dissected with the knife or the CUSA the way the operator wishes. But, the formalized cadaver brain will break into strips and small chunks even to the strokes of the sharpest knife. Also, in the living brain one might see emission of CSF as soon as the temporal horn ependyma is breached; the choroid too, might peek through. The cadaver brain ventricle will not show CSF emission and the choroid is plastered to the inner surface of the ventricle. So an inexperienced trainee might enter the temporal horn and will go through to the opposite wall even without noticing. This is true whether the ventricle was reached with the naked eye vision or with the loupes. Even with the dissecting microscope there is a chance of missing the ventricle due to a difficult retraction. Therefore, a stained ventricle wall will no doubt help the dissector confirming that he has entered the ventricle. Thus, in our center we developed a technique to stain the ventricles. We also use the same technique when a brain specimen is dissected and photographed for teaching purposes. In our series all specimens stained as expected. However, if there is an unforeseen obstruction within the ventriclular cavity due to a tumor or some adhesion, the stain may not travel far enough to produce the expected result. Another drawback is when the specimen is retransferred to and from the formalin bath several times in between dissections, a significant portion of the stain is lost over the first month. This study was approved by the medical school research committee. The specimens were donated by citizens supporting medical research. Therefore no external funding was involved. The items needed are, a formalized cadaver brain with an intact pia (harvested out of the cranium), a bottle of red ink (fountain pen ink), a 20cc syringe and an 18 gauge needle.
On the basal surface of the brain one can visualize the circle of Willis, with the open ends of the internal carotid and vertebral/basilar arteries. Veins are collapsed in most of the specimens, but could be identified beneath the arachnoid. Pure red ink is injected with some force to the open ends of each artery. Sometimes intra-arterial clots will limit the efficiency of this process. One could see that the ink start to leak from the unidentified torn vascular openings and will start to stain the basal surface of the brain. This may even alarm the dissector with the thought that the specimen will over stain. If this happens hold the entire brain under running water to remove excess ink.
Following this, the user should inject a few milliliters of ink into the 3rd ventricle through its floor. The brain should be kept upside down so that the ink will travel well to the lateral ventricles. Then rock the brain sideways for 4 to 5 minutes for the ink to spread uniformly inside the lateral ventricles. Now turn the brain specimen to its correct orientation. Then the excess red ink will dribble from the 3rd ventricular opening and from the 4th ventricle. Immediately transfer the specimen to the sink and leave it under running water to take away the excess stain. This is important as at this stage any excess stain will turn the brain surface to a red globe. This is because excess ink will seep under the breeched pia. Allow the excess water to drip into the sink. The specimen is then transferred to the usual 10% formalin container. Leave it there for 24 hours. After 24 hours take the specimen out of the bath and allow the excess formalin to drip away. If the formalin vapor is too strong use running water for 2-3 minutes to neutralize the effect. Now the brain specimen is ready for dissection. The dissector will notice that although the brain surface has turned pink (and not red), it is the pia which is stained. Carefully remove the pia in the area that is subjected to dissection. Then the natural dusky-white color of the cadaver brain will reappear. Now the dissector can start the dissection. Specimen is dissected starting from the outer surface and working towards the ventricles. As soon as the dissection enters the ventricle a dark pink color is noted. The dissector will appreciate that most of the time the ventricle is collapsed and without the stain he would have easily gone through the ventricle and even cut through the opposite wall. He will also notice that the choroid plexus is collapsed and pasted to the ventricular surface, but has a glowing pinkish granular appearance. Choroid is not well seen in the non-stained specimen. Beyond this point the dissector will need the dissecting microscope. If the specimen is used for photo documentation the lateral wall is opened widely to get a better view.
Three investigators independantly evaluated the results. Six brain specimens were used (twleve temporal horns). Three brains were stained and three were not. Each investigator had to approach the temporal horn of a non-stained specimen followed by an approach to the temporal horn of a stained specimen. Then the sequence was repeated once more on fresh specimens. One dissector was able to identify the 2nd non-stained temporal horn without much difficulty. Even then, at the ventricular entry point he cut through the unstained choroid that was plastered to the lateral wall. This form of testing was not validated and was purely observational. No statistics were pertinent to this study. The photographs displayed here are selected from a series of an approach to temporal horn. In these specimens a wider window is made for the purpose of clear demonstration; usually a dissector uses a smaller window. There is a difference in color in side ventricle, parenchyma and the brain stem(Fig. -1), (Fig. -1) . The arteries near the brain stem are visualized in a glistening purple (when a thrombus is inside) and glistening red (when the lumen is empty)(Fig. -1). Figure 1: Interior of the temporal horn
 |
| Stained interior of the temporal horn(1), Unstained parenchyma(2), Dissected hippocampus tail(3), Tela choroidea(4), Brain stem visualized through choroid fissure(5), Choroid plexus(6). |
Figure 2: Close-up view of intraventricular contents
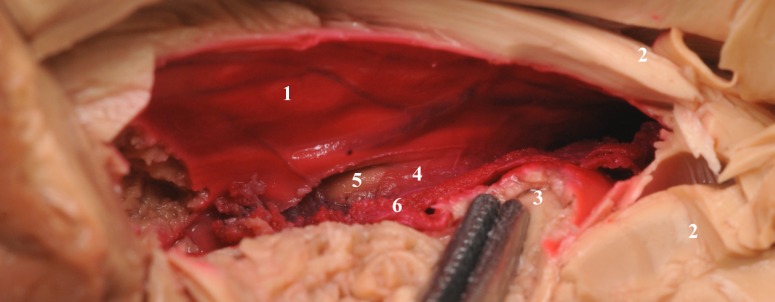
 |
| Internal digitations of head of hippocampus(1), Choroid plexus(2), A view through choroid fissure(3), Unstained temporal lobe parenchyma(4). |
Figure 3: A view through choroid fissure
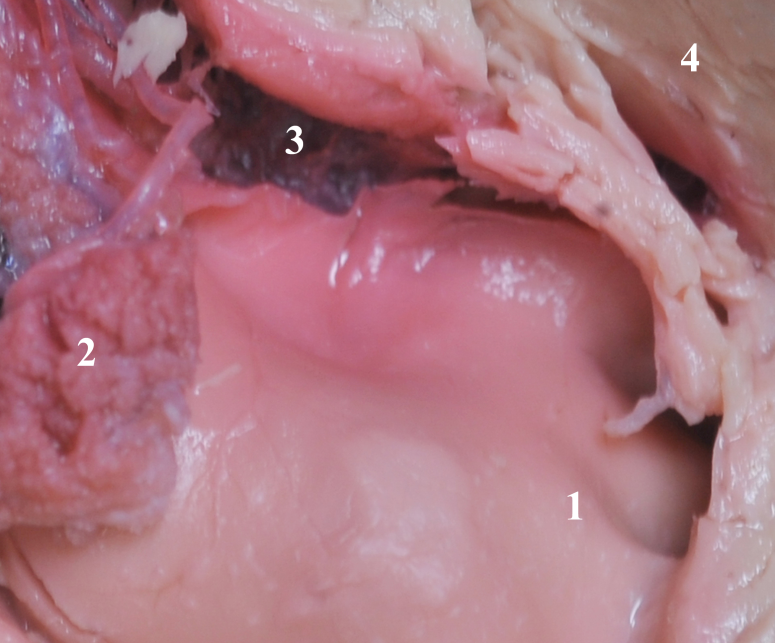
 |
| Posterior cerebral artery and its branches. Note that the segment of the vessel harboring a thrombus appears purple and without a thrombus appear red. |
Many approaches are used to access the ventricular system to treat various pathological conditions5,10,11,13 . Hence, mastering the techniques to enter the ventricular system are an important aspect of neurosurgical training. Brain specimens from human cadavers or from other animals are used for this purpose9 . In this laboratory report we are recording a technique that is used in our center for ventricular staining of formalized cadaver brain. It is simple, inexpensive and easy to perform. The results are consistent and meaningful to the trainee. Most of the staining techniques described for cadaver brain are meant for microscopic studies. Even the macroscopic staining methods that are designed for cadaver brain slices, are meant to highlight the brain parenchyma8,2,3,7,12,13 . Most of these techniques are used to differentiate gray and white matter1,4 . Our technique is different in that it is used to identify the ventricular system and its contents. In our center we use 10% formalin as a preservative and keep the specimen in the solution for 2 months. We do not use the freezing method used in other centers. Then we subject these cadaver specimens to the staining method described above.
The staining technique described here will highlight the ventricular system. It should help the dissector who is practicing a cortical approach to the ventricles. This is especially true when a dissector starts his training session with a naked eye or loupe vision. It could also be used to demonstrate the intraventricular contents in photo recording in descriptive brain anatomy.
1. Barnett, R.,Burland, G.,Duxson, M., Plastination of Coronal Slices of Brains from Cadavers using the P35 Technique. Journal of International Society for Plastination 20: 16 - 19, 20052. Braak, H., Simple and Durable Staining of Thick Sections of the Human Brain for Macroscopic Study. Biotechnic and Histochemistry 53(2): 87 - 89, 19783. Brown, D., A simple macroscopic staining and mounting procedure for wet sections from cadaver brains. The Anatomic Record 75(4): 419 - 425, 2005 February4. Duvernoy, H., The Human Hippocampus (Chapter 2, Material and Methods). Third Edition. Springer. 2005.5. Ellenbogen, R., Transcortical surgery for lateral ventricular tumors. Neurosurg Focus 10(6): - , 20016. Heimer, L., Gross dissection of human brain. Neurosurg Focus 18(6b): 1 - 2, 20057. Heller, M.,Stoddard, S., Procedure for Staining Fixed Human Brain Slices. Biotechnic and Histochemistry 61(2): 71 - 73, 19868. Hewitt, W., A method for staining whole brain for gross and macroscopic study. Journal of Anatomy 93(1): 134 - 136, 1959 January9. Hicdonmez, T.,Hamamcioglu, M.,Parsak, T.,Cukur, Z.,Cobanoglu, S., A laboratory training model for interhemispheric-transcallosal approach to the lateral ventricle. Neurosurg Rev. : - , 200510. Ojemann, R., Intraventricular Meningioma. Clinical Neurosurgery 40 (17): 321 - 383, 199211. Pamir, M.,Black, P.,Fahlbusch, R., Meningioma - A Comprehensive Text - Chapter 45 (Intraventricular Meningioma - A. Goel et al, 561-567). First Edition. Elsevier. 2010.12. Riboni, L.,Luna, F.,Nunez-Duran, H., A fast staining method for CNS slices. Journal of Neuroscience Methods 38(2): 239 - 241, 1991 July13. Roberts, M.,Hanaway, J., Preperation of Brain Slices for Macroscopic Study by the Copper Sulphate-Phenol-Ferrocyanide Technique. Biotechnic and Histochemistry 44(3): 143 - 146, 196914. Yasargil, M.,Ture, U.,yasargill, D., Surgical anatomy of supratentorial midline lesions. Neurosurgery Focus 18(6b): - , 2005 June
|

